West Pharma launches packaging component AccelTRA for generic drug manufacturers in India
In order to meet global regulatory norms, US based pharmaceutical packaging company West Pharma has launched new packaging component AccelTRA for generic drug manufacturers in injectable category in India.
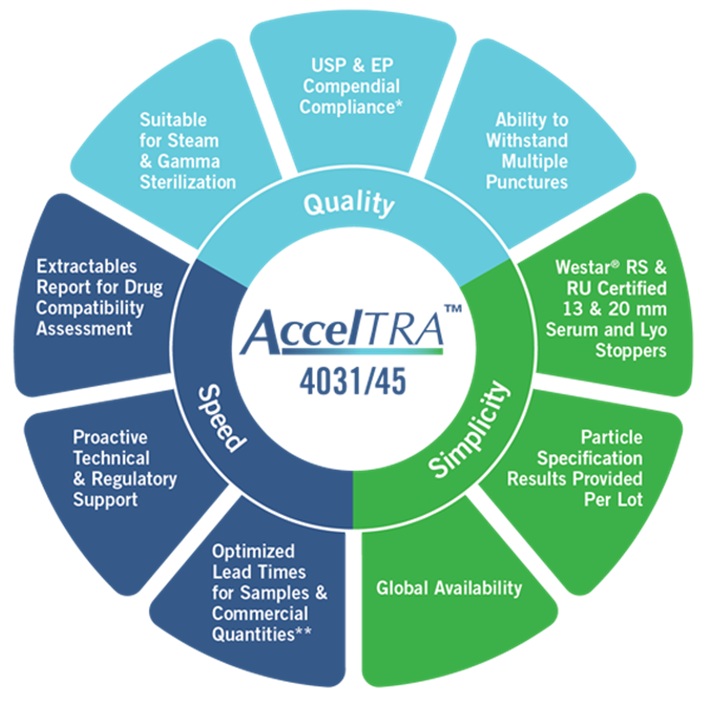
AccelTRA offers customers a rubber formulation compatible with the needs of generic drug manufacturers seeking quality, speed and simplicity.
The pharma packaging industry in India is evolving rapidly to adopt to changing policies and regulations in their markets as many Indian companies are now moving into the US and Europe.
Elaborates Fran DeGrazio, vice president, global scientific affairs and technical services, West Pharma, “The company is working with many local generic companies in India. We realised that these generic manufacturers are under considerable pressure to get their products to market quickly within a stricter regulatory environment. That’s why we launched AccelTRA component programme in the local market, which delivers packaging quality for them.”
DeGrazio further explains, “Often generic drug manufacturers may seek to use the same formulation as the innovator drug. However, those formulations may have been developed in the 1970s or 80s, meaning that they may not be as advanced as more modern formulations, which could be a disadvantage to compatibility with today’s wide range of generic molecules.”
AccelTRA offers a high-quality formulation with a short lead time and regulatory support. This is just one area that the company is currently focusing on.
“In India and around the world, there are a number of trends driving the need for innovation and quality in injectable drug packaging and delivery. These include growth in generics and biosimilars as well as biologics and an increasing demand for combination products. In addition, concern for patient safety has led regulatory agencies to ask drug and packaging manufacturers to build quality into their products from the start, resulting in a move toward the use of a Quality by Design (QbD) approach to manufacturing,” says while talking about the trends in trends in injectable drug packaging.
One of the biggest challenges faced by pharmaceutical and biopharmaceutical companies and their drug delivery system partners is navigating the changes in global regulatory requirements.
As regulations increase across the pharmaceutical industry, reliable scientific evidence is required to support the decisions being made during the drug development process in order to achieve the safe delivery of medicines to patients. Recent regulations around issues such as particulate and heavy metals, require a strong understanding of the testing and analysis to move drug products to the market.
“For example, particulates in injectable drug products continue to be an area of great interest in the industry. The main reason is the potential clinical effects on patients which can range from emboli to inflammations to infections. There are a series of clinical risk factors that should be considered in evaluating and mitigating these challenges. These factors are route of administration, patient population, and particulate composition (i.e., number, size, and shape). All are critical inputs to understanding risk and determining corrective and preventative actions,” she adds.
West’s Integrated Solutions programme brings together primary packaging, device, analytical, regulatory and contract manufacturing expertise in a single-source package that is designed for any stage of the drug development life-cycle and across all injectable formats.
In order to achieve further improvement, it is critical to understand testing approaches and all factors that could impact particulate formation and drug product contamination. At West, particulates are a thrust area. The company has the expertise and facility not only to measure particulate levels and types precisely but is also actively engaged in developing new measurement methods.
“Biological companies may not have complete in-house capability from development to analytical studies to filling and labelling. We can partner with them on pre-screening i.e. initial development of the packaging for them. Once they move from formulation testing to packaging, such as in a pre-fillable syringe, cartridges or vials, we can help them to identify the right packaging and delivery components and systems suitable for both the drug and the patient,” DeGrazio concludes.
Source : pharmabiz