Illegal cancer drugs from Bangladesh flood local market

It’s not just import of raw materials (active pharmaceutical ingredients, or APIs) from China that is giving sleepless nights to domestic pharma companies. Now, “illegal” import of drugs from Bangladesh and other neighbouring countries have added to their worries. These not only impact revenues but, more importantly, pose a risk to patients.
Studies undertaken by experts, and confirmed by companies, suggest a thriving ‘grey’ market of fake and unapproved copies of Big Pharma’s oncology and hepatology medicines, and some under patent protection. Since these medicines are smuggled, exact numbers are unavailable, but estimates suggest that this grey market could be over Rs 300 crore for just oncology drugs. About 12% of those prescribed these medications could be consuming fake tablets/capsules, according to oncologists. The safety and efficacy of these capsules is not known, as they are not imported through the legal route.
Further, these drugs have not undergone clinical trials, and do not have the drug controller’s approvals. It is understood that quasi-government bodies — like the Employees’ State Insurance Corporation (ESIC) and the Central Government Health Scheme (CGHS) — are unknowingly sourcing these products. Sources said that the Organisation of Pharmaceutical Producers of India (OPPI) recently discussed the matter with the government. The member companies were assured that some steps would be taken soon. “A majority of these medicines have been manufactured by companies in Bangladesh. They are made only for exports. It would help if the border is strengthened so as to prevent the illegal entry of these drugs,” an expert said.
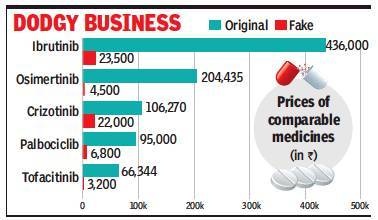
Unlike other drugs, cancer drugs are not sold through retail chemists, but by distributors. So it would be relatively easier to trace those involved. MNCs hit due to the thriving grey market include Novartis, Janssen (J&J’s pharma arm), Astra Zeneca, Takeda and Eisai. This is because copies of drugs like osimertinib priced around Rs 2 lakh by Astra Zeneca, crizotinib by Pfizer with a price tag of over Rs 1 lakh, and ibrutinib by Janssen, priced at over Rs 4 lakh in the domestic market, are available at a fraction of the price (see graphic).
A Janssen spokesperson said, “Whenever we are alerted to potential illegal imports, we work with relevant judicial authorities and law enforcement agencies to attempt to combat such activity.” A study in Indian Journal of Medical Sciences says certain Bangladesh companies are blatantly targeting doctors and patients from across the border using WhatsApp, email, social media and, in certain cases, are reaching out to patients through chemists located near cancer hospitals.
Purvish Parikh, an oncologist with Shalby Cancer and Research Institute, and the study’s co-author, said, “The package boxes are of good quality and can entice even the educated into believing that its contents are also produced with care. However, the batch numbers and bar codes do not follow internationally accepted norms. When it is coupled with the fact that there is no evidence of any testing in humans, no drug authority/ regulatory approval, not marketed within Bangladesh itself, no official presence in India, there is a serious question on whether the package contains simply an inert powder or, still worse, chemicals of questionable quality that could potentially be lethal.”
Further, Eisai Pharma MD Sanjit Singh Lamba said, “Bar coding on medicine packs could prevent this. The government has announced bar coding for domestic sales — which is voluntary right now. But we have urged it to be made mandatory (on manufacturers).” Most associations like OPPI, IPA and IDMA favour a secure supply chain for pharma products, he added.
“Traders smuggle unapproved medicines and sell it in India illegally. Some counterfeits/fakes are available at 1/20th of the innovators’ products. There is a definite compromise on the expected treatment outcome when such unapproved medicines are used,” said Praveen Sikri, founder and CEO of Ikris Pharma Network, which connects patients with overseas suppliers.
Source : economictimes.indiatimes