FDA Accepts Sanofi’s BLA for Meningitis Vaccine to Review
Sanofi SNY announced that the FDA has accepted for review its biologics license application (BLA) for MenQuadfi, a meningococcal vaccine candidate, currently being developed for the prevention of meningococcal meningitis. The regulatory body has set an action date of Apr 25, 2020. On approval, the MenQuadfi vaccine will be available in a fully liquid presentation.
The BLA was based on positive data from the phase II/III studies, seeking an approval for use of the meningococcal (Groups A, C, Y, W) vaccine in patients aged two years and above.
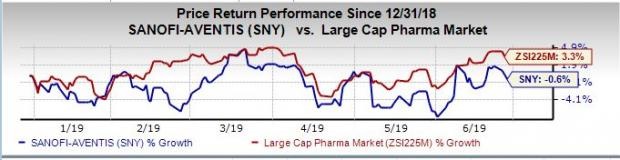
Shares of Sanofi have dipped 0.6% so far this year against the industry’s rise of 3.3%.
Sanofi conducted the studies across the United States, Europe, Asia and Latin America. Additional phase III studies are also on course by the company in Africa. The ongoing program includes subjects of different ages, varying from infants aged 6 weeks to older adults. The overall objective of this evaluation is to assess the vaccine’s ability to help prevent meningococcal meningitis, a rare but serious bacterial infection, in patients across a broad range of age groups.
We remind investors that Sanofi possesses one of the world’s leading vaccine operations. Apart from pediatric vaccines, the company’s portfolio includes influenza vaccines, adult and adolescent booster vaccines, meningitis vaccines plus travel and endemic vaccines. Sanofi also holds a strong position in both seasonal and pre-pandemic influenza vaccine spaces.
Last December, the FDA approved Sanofi’s pediatric vaccine Vaxelis, which has been developed for active immunization to prevent six different diseases in children aged six weeks to four years.
Vaxelis has been jointly developed by Sanofi and Merck MRK . Both companies are working on the vaccine production with a commercial launch expected not before 2020.
Notably, last November, the European Commission granted a marketing authorization to Sanofi’s dengue vaccine, Dengvaxia. The vaccine is already available across 20 countries for the prevention of dengue.
Total sales of the company’s vaccine division, Sanofi Pasteur, in the first quarter of 2019 (including the emerging markets) grossed 873 million euros, reflecting a 20.1% increase, driven by a strong performance of its Polio/Pertussis/Hib vaccines in the emerging markets and Japan.
Sanofi is focused on fortifying its vaccine business further.
Meanwhile, last week, Sanofi entered into a collaboration contract with Google, a subsidiary of Alphabet Inc. GOOG , to establish a new virtual healthcare innovation laboratory for transforming future medicines and healthcare services with the aid of latest data technologies.
With this partnership, Sanofi is looking to gain a better understanding of patients and their diseases as well as improve its patients’ and customer experience. The company is also eyeing to enhance operational efficiency while developing new therapies via emerging data technologies.
Zacks Rank & Stock to Consider
Sanofi currently carries a Zacks Rank #3 (Hold). A better-ranked stock in the large cap pharma sector is Novartis AG NVS , which sports a Zacks Rank #1 (Strong Buy). You can see the complete list of today’s Zacks #1 Rank stocks here .
Novartis’ earnings estimates have moved 0.6% north for 2019 and 1.5% for 2020 over the past 60 days. The stock has gained 6.3% year to date.
Breakout Biotech Stocks with Triple-Digit Profit Potential
The biotech sector is projected to surge beyond $775 billion by 2024 as scientists develop treatments for thousands of diseases. They’re also finding ways to edit the human genome to literally erase our vulnerability to these diseases.
Zacks has just released Century of Biology: 7 Biotech Stocks to Buy Right Now to help investors profit from 7 stocks poised for outperformance. Our recent biotech recommendations have produced gains of +98% , +119% and +164% in as little as 1 month. The stocks in this report could perform even better.
Source : nasdaq