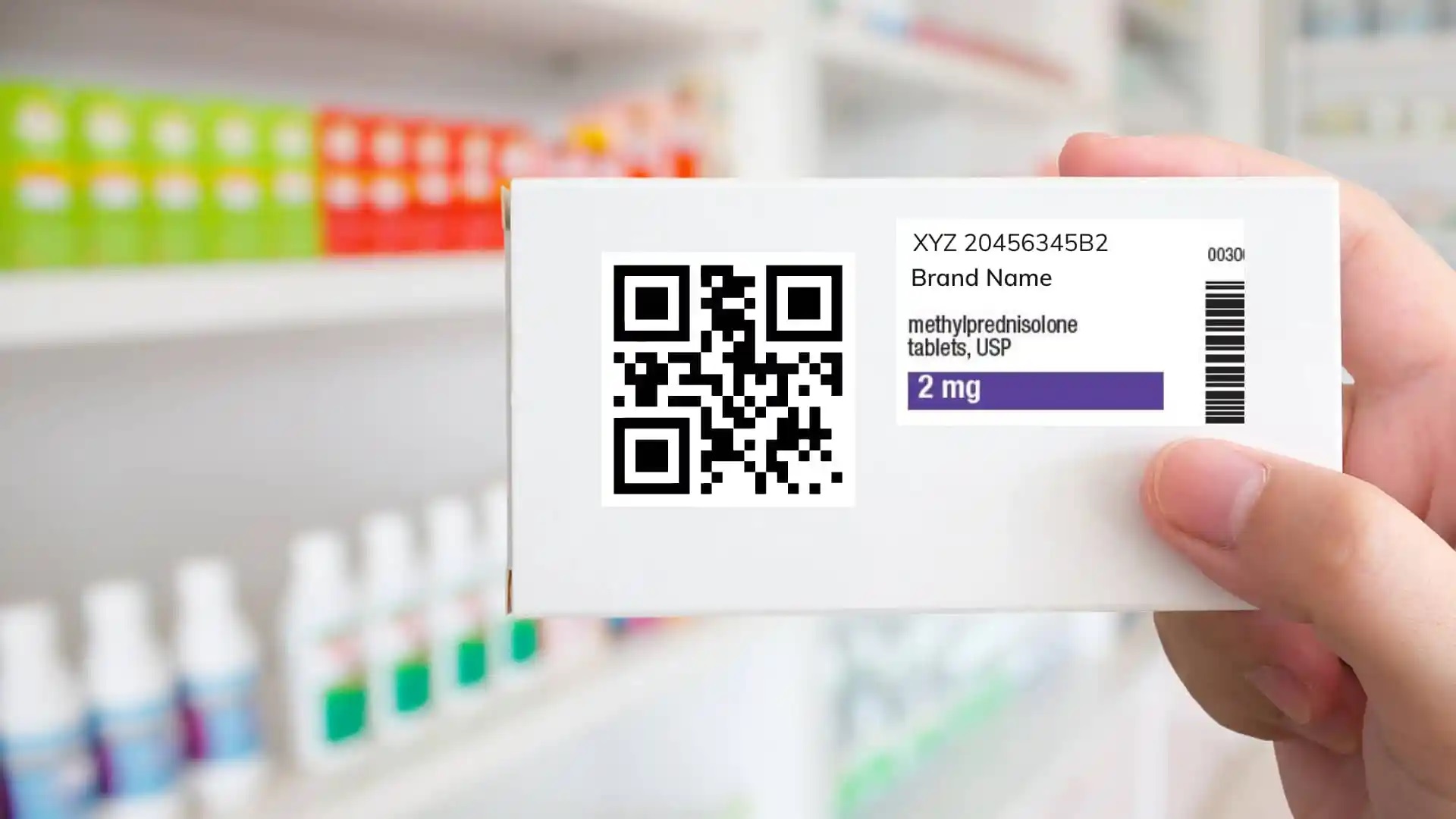
The Union health ministry has notified an amendment to the Drugs Rules, 1945 to mandate inclusion of qualitative details of excipient into the data stored in the label of drug formulation products as bar code or Quick Response (QR) code from next year.
The final notification was issued as the Drugs (2nd Amendment) Rules, 2025, which shall come into force from March 1, 2026.
While the draft notification issued in July 12, 2024, proposed to add meagerly the “details of excipients” to the sub-rule (7) of the Rule 96, into to the list of details to be incorporated in the QR code, the final notification went one step ahead and stipulated to add the “qualitative details of excipients” into the QR code.
The move is expected to rectify problems including those suffered by people allergic to certain excipients, since they or their medical practitioners can identify the kind of excipients used in a drug formulation before its use.
According to the Drugs Rules, the manufacturers of drug formulation products as specified in Schedule H2 shall print or affix bar code or QR code on its primary packaging label or, in case of inadequate space in primary package label, on the secondary package label that store data or information legible with software application to facilitate authentication.
The sub-rule (7) of Rule 96 lists out the data to be stored in the QR code or bar code. This include the unique product identification code, proper and generic name of the drug, brand name, name and address of the manufacturer, batch number, date of manufacturing, date of expiry, and manufacturing license number. The qualitative data of the excipients has now been added as the ninth data to be stored in the QR code, from next March onwards.
In the final notification, the ministry of health said that the objections and suggestions received from the public on the draft rules, issued on July 12, 2024, have been considered by the Central government.
As reported earlier, the Drugs Technical Advisory Board (DTAB) of the Ministry had earlier considered the proposal for inclusion of the name of excipients in the label, following a grievance received that the parabens are used in pharmaceutical products and preservatives, as excipients and many people are allergic to paraben and other excipients.
There is no clear cut indication of composition of excipient on the strips of medicines available on retail medical shops and patients find it difficult to find paraben free antihypertensive medicines and others. The suggestion was to include the details of excipient or INS codes of the excipients on every strip of medicines.
The proposal was deliberated in the 61st Drugs Consultative Committee (DCC) meeting where the committee deliberated the matter that details of the excipients should be in the package inserts of the medicines. It observed that presently there is no provision which makes it mandatory for the manufacturers to provide package inserts along with the drugs manufactured/marketed in the country. The criteria to mandate mentioning of the details of excipients on drug formulations have to be evaluated at length for its implementation, it said.
Considering overall perspective, the committee after detailed deliberation recommended issuing an advisory for mentioning details of excipients on drug formulation by various means/modality on a voluntary basis.
However, DCC in its 62nd meeting held in September, 2023 while reviewing the Action Taken Report of 61st DCC recommended that mentioning of all the excipients on the product label is a practical challenge and there is no mandatory requirement.
DCC deliberated and suggested for capturing this information through the QR code or by capturing this information in the package insert. The Committee recommended that the notification issued on November 17, 2022 mandating barcode or QR code for all top 300 brands may be amended for capturing the requisite information in the QR code at least for these top 300 brands initially.
DTAB, in its meeting held on January 25, 2024, deliberated the matter and opined that it is difficult to include the details of all excipients on every strip of medicines. Further the Board also suggested preparing a list of excipients causing hypersensitivity which may be considered for mention on the label.
“However, DTAB agreed for the proposed amendment with regard to capturing the requisite information in QR code for top 300 brands,” it added.
Source : Pharmabiz